Este iFluor® 488 tiene propiedades espectrales y reactividad similares al éster Alexa Fluor® 488 NHS (Alexa Fluor® es una marca comercial de Invitrogen).
Descripción
Aunque FITC sigue siendo el tinte marcador fluorescente más popular para la preparación de bioconjugados fluorescentes verdes, existen ciertas limitaciones con FITC, como fotoblanqueo severo para imágenes microscópicas y fluorescencia sensible al pH. derivados de fluoresceína como FITC. Los conjugados iFluor® 488 son significativamente más brillantes que los conjugados de fluoresceína y son mucho más fotoestables. Además, la fluorescencia de iFluor® 488 no se ve afectada por el pH (4-10), que emite su máxima fluorescencia solo a pH por encima de 9.
El tinte iFluor® 488 SE es razonablemente estable y muestra una buena reactividad y selectividad con los grupos amino de la proteína. Este iFluor® 488 tiene propiedades espectrales y reactividad similares al éster Alexa Fluor® 488 NHS (Alexa Fluor® es la marca comercial de Invitrogen).
| Catalogo | Producto | Presentación |
|---|---|---|
| AAT-1023 | iFluor® 488 succinimidyl ester | 1mg |
| AAT-71023 | iFluor® 488 succinimidyl ester | 100 ug |
| AAT-71503 | iFluor® 488 succinimidyl ester | 5mg |
| AAT-71553 | iFluor® 488 succinimidyl ester | 10mg |
Importante: Solo para uso en investigación (RUO). Almacenamiento: Congelación (< -15 °C). Minimizar la exposición a la luz.
Propiedades fisicas
| Peso Molecular | 945.07 |
| Disolvente | DMSO |
Espectro
Abrir en Advanced Spectrum Viewer

Propiedades espectrales
| Factor de corrección (260 nm) | 0.21 |
| Factor de corrección (280 nm) | 0.11 |
| Coeficiente de extinción (cm -1 M -1) | 750001 |
| Excitación (nm) | 491 |
| Emisión (nm) | 516 |
| Rendimiento cuántico | 0.91 |
Calculadora
Preparación de la solución de stock común
Volumen de DMSO necesario para reconstituir la masa específica de succinimidil éster iFluor® 488 a la concentración dada. Tenga en cuenta que el volumen es solo para preparar la solución madre. Consulte el protocolo experimental de la muestra para conocer los buffers experimentales/fisiológicos apropiados.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 105.812 µL | 529.061 µL | 1.058 mL | 5.291 mL | 10.581 mL |
| 5 mM | 21.162 µL | 105.812 µL | 211.625 µL | 1.058 mL | 2.116 mL |
| 10 mM | 10.581 µL | 52.906 µL | 105.812 µL | 529.061 µL | 1.058 mL |
Imagenes

Figura 1. Las células HeLa se tiñeron con antitubulina de conejo seguida de iFluor 488 de cabra anti-IgG de conejo (H+L), y los núcleos se tiñeron con Nuclear Red DCS1.

Figura 2. Análisis de citometría de flujo de PBMC teñidas con iFluor® 488 conjugado anti-CD24 humano *HI45*. La señal de fluorescencia se controló utilizando un citómetro de flujo Aurora en el canal B2-A específico de iFluor® 488.
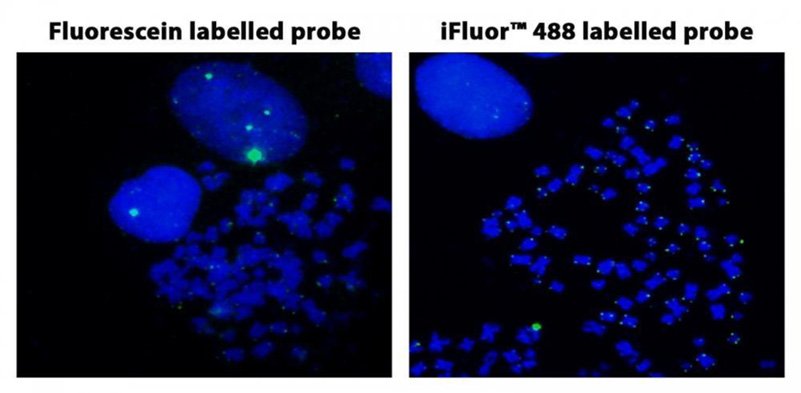
Figura 3. Hibridación in situ con fluorescencia de sondas de telómero marcadas con fluoresceína y iFluor® 488 en células HeLa en metafase.

Figura 4. Análisis de citometría de flujo de Alexa Fluor® 488 o iFluor® 488 anti-CD4 humano en linfocitos humanos. Las células de PBMC se tiñeron con 0,5 ug de Alexa Fluor® 488 anti-CD4 humano o 0,5 ug de iFluor® 488 anti-CD4 humano en cada prueba. El análisis de citometría de flujo se realizó en un sistema de citometría de flujo ACEA.
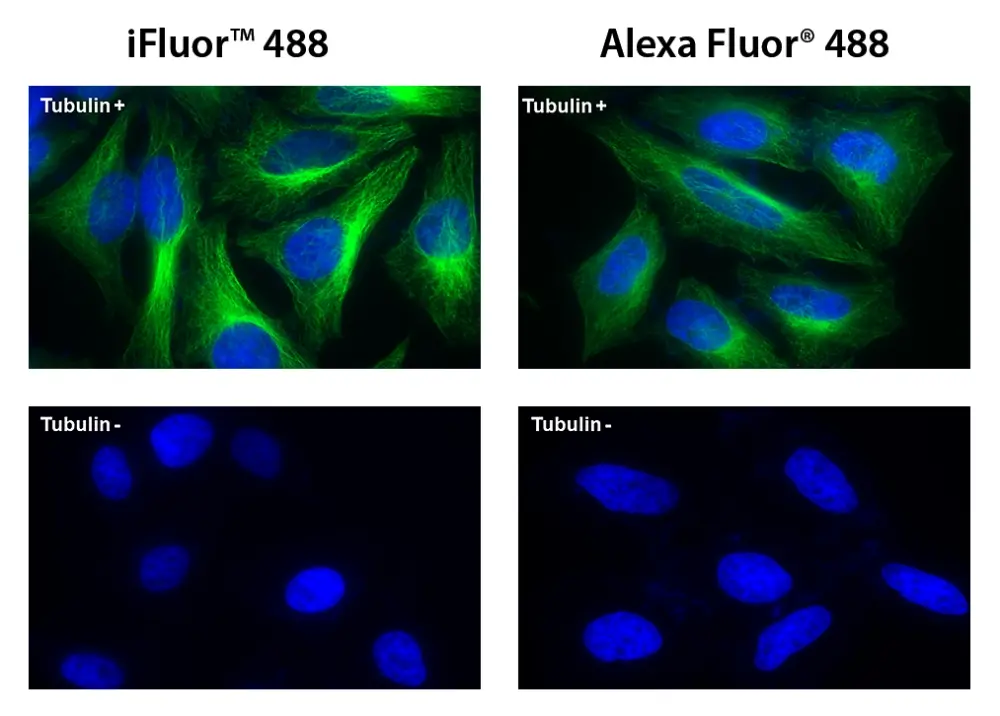
Figura 5. Las células HeLa se incubaron con (Tubulin+) o sin (Tubulin-) antitubulina de ratón seguido de iFluor® 488 conjugado de IgG anti-ratón de cabra (Verde, izquierda) o Alexa Fluor® 488 conjugado de IgG anti-ratón de cabra (Verde , Derecha), respectivamente. Los núcleos celulares se tiñeron con Hoechst 33342 (azul).
Productos Similares
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 succinimidyl ester | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 405 succinimidyl ester | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| iFluor® 514 succinimidyl ester | 511 | 527 | 750001 | 0.831 | 0.265 | 0.116 |
| iFluor® 532 succinimidyl ester | 537 | 560 | 900001 | 0.681 | 0.26 | 0.16 |
| iFluor® 555 succinimidyl ester | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| iFluor® 594 succinimidyl ester | 588 | 604 | 1800001 | 0.531 | 0.05 | 0.04 |
| iFluor® 633 succinimidyl ester | 640 | 654 | 2500001 | 0.291 | 0.062 | 0.044 |
| iFluor® 647 succinimidyl ester | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| iFluor® 660 succinimidyl ester | 663 | 678 | 2500001 | 0.261 | 0.07 | 0.08 |
Bibliografía
Ver todas las 32 bibliografías: Citation Explorer
Kukoamine A attenuates lipopolysaccharide-induced apoptosis, extracellular matrix degradation, and inflammation in nucleus pulposus cells by activating the P13K/Akt pathway
Authors: Wang, Dan and Qu, Hao and Kang, Hui and Xu, Feng and Huang, Wei and Cai, Xianhua
Journal: Bioengineered (2022): 8772–8784
Evaluation of Usnea barbata (L.) Weber ex FH Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy
Authors: Popovici, Violeta and Matei, Elena and Cozaru, Georgeta Camelia and Bucur, Laura and G{\^\i}rd, Cerasela Elena and Schr{\”o}der, Verginica and Ozon, Emma Adriana and Karampelas, Oana and Musuc, Adina Magdalena and Atkinson, Irina and others,
Journal: Antioxidants (2022): 1601
Independent phenotypic plasticity axes define distinct obesity sub-types
Authors: Yang, Chih-Hsiang and Fagnocchi, Luca and Apostle, Stefanos and Wegert, Vanessa and Casan{\’\i}-Gald{\’o}n, Salvador and Landgraf, Kathrin and Panzeri, Ilaria and Dror, Erez and Heyne, Steffen and W{\”o}rpel, Till and others,
Journal: Nature metabolism (2022): 1150–1165
ZBTB7A promotes virus-host homeostasis during human coronavirus 229E infection
Authors: Zhu, Xinyu and Trimarco, Joseph D and Williams, Courtney A and Barrera, Alejandro and Reddy, Timothy E and Heaton, Nicholas S
Journal: Cell Reports (2022): 111540
Osteoclasts directly influence castration-resistant prostate cancer cells
Authors: Huang, Junchi and Freyhult, Eva and Buckland, Robert and Josefsson, Andreas and Damber, Jan-Erik and Wel{\’e}n, Karin
Journal: Clinical \& experimental metastasis (2022): 801–814
Phase separation of insulin receptor substrate 1 drives the formation of insulin/IGF-1 signalosomes
Authors: Gao, Xiu Kui and Rao, Xi Sheng and Cong, Xiao Xia and Sheng, Zu Kang and Sun, Yu Ting and Xu, Shui Bo and Wang, Jian Feng and Liang, Yong Heng and Lu, Lin Rong and Ouyang, Hongwei and others,
Journal: Cell discovery (2022): 1–19
Arabidopsis cryptochrome 2 forms photobodies with TCP22 under blue light and regulates the circadian clock
Authors: Mo, Weiliang and Zhang, Junchuan and Zhang, Li and Yang, Zhenming and Yang, Liang and Yao, Nan and Xiao, Yong and Li, Tianhong and Li, Yaxing and Zhang, Guangmei and others,
Journal: Nature communications (2022): 1–15
Arc weakens synapses by dispersing AMPA receptors from postsynaptic density via modulating PSD phase separation
Authors: Chen, Xudong and Jia, Bowen and Araki, Yoichi and Liu, Bian and Ye, Fei and Huganir, Richard and Zhang, Mingjie
Journal: Cell Research (2022): 914–930
A Novel CDK4/6 and PARP Dual Inhibitor ZC-22 Effectively Suppresses Tumor Growth and Improves the Response to Cisplatin Treatment in Breast and Ovarian Cancer
Authors: Tian, Chenchen and Wei, Yufan and Li, Jianjun and Huang, Zhi and Wang, Qiong and Lin, Yingxue and Lv, Xingping and Chen, Yanan and Fan, Yan and Sun, Peiqing and others,
Journal: International journal of molecular sciences (2022): 2892
p73-regulated FER1L4 lncRNA sponges the oncogenic potential of miR-1273g-3p and aids in the suppression of colorectal cancer metastasis
Authors: Uboveja, Apoorva and Satija, Yatendra Kumar and Siraj, Fouzia and Saluja, Daman
Journal: Iscience (2022): 103811
Referencias
Ver todas las 49 referencias: Citation Explorer
Sequential ordering among multicolor fluorophores for protein labeling facility via aggregation-elimination based beta-lactam probes
Authors: Sadhu KK, Mizukami S, Watanabe S, Kikuchi K.
Journal: Mol Biosyst (2011): 1766
Visualizing dengue virus through Alexa Fluor labeling
Authors: Zhang S, Tan HC, Ooi EE.
Journal: J Vis Exp. (2011)
Fluorescent “Turn-on” system utilizing a quencher-conjugated peptide for specific protein labeling of living cells
Authors: Arai S, Yoon SI, Murata A, Takabayashi M, Wu X, Lu Y, Takeoka S, Ozaki M.
Journal: Biochem Biophys Res Commun (2011): 211
Neuroanatomical basis of clinical joint application of “Jinggu” (BL 64, a source-acupoint) and “Dazhong” (KI 4, a Luo-acupoint) in the rat: a double-labeling study of cholera toxin subunit B conjugated with Alexa Fluor 488 and 594
Authors: Cui JJ, Zhu XL, Ji CF, Jing XH, Bai WZ.
Journal: Zhen Ci Yan Jiu (2011): 262
Simultaneous detection of virulence factors from a colony in diarrheagenic Escherichia coli by a multiplex PCR assay with Alexa Fluor-labeled primers
Authors: Kuwayama M, Shigemoto N, Oohara S, Tanizawa Y, Yamada H, Takeda Y, Matsuo T, Fukuda S.
Journal: J Microbiol Methods (2011): 119
Alexa Fluor 546-ArIB[V11L;V16A] is a potent ligand for selectively labeling alpha 7 nicotinic acetylcholine receptors
Authors: Hone AJ, Whiteaker P, Mohn JL, Jacob MH, McIntosh JM.
Journal: J Neurochem (2010): 994
Asymmetric trimethine 3H-indocyanine dyes: efficient synthesis and protein labeling
Authors: Song F, Wang L, Qiao X, Wang B, Sun S, Fan J, Zhang L, Peng X.
Journal: Org Biomol Chem (2010): 4249
Neuroanatomical characteristics of acupoint “Chengshan” (BL 57) in the rat: a cholera toxin subunit B conjugated with Alexa Fluor 488 method study
Authors: Zhu XL, Bai WZ, Wu FD, Jiang J, Jing XH.
Journal: Zhen Ci Yan Jiu (2010): 433
Photoactivatable and photoconvertible fluorescent probes for protein labeling
Authors: Maurel D, Banala S, Laroche T, Johnsson K.
Journal: ACS Chem Biol (2010): 507
Novel Alexa Fluor-488 labeled antagonist of the A(2A) adenosine receptor: Application to a fluorescence polarization-based receptor binding assay
Authors: Kecskes M, Kumar TS, Yoo L, Gao ZG, Jacobson KA.
Journal: Biochem Pharmacol (2010): 506
Application Notes
FITC (Fluorescein isothiocyanate)
Fluorescein isothiocyanate (FITC)
iFluor® Dye Selection Guide
Introducing Calbryte™ Series
FITC (Fluorescein isothiocyanate)
FAQ
What are common laser lines used in flow cytometry?
What are the spectral properties of iFluor dyes?
Why should isotype controls be used in immunofluorescence staining?
Are any of the cyanine dyes infrared?
Are coumarin dyes pH sensitive?


